ᱦᱟᱭᱤᱰᱨᱚᱡᱟᱱ ᱥᱟᱞᱯᱷᱟᱭᱤᱰ
Appearance

| |||
| |||
| Names | |||
|---|---|---|---|
| ᱥᱤᱥᱴᱟᱢᱮᱴᱤᱠ IUPAC ᱧᱩᱛᱩᱢ
Hydrogen sulfide[᱑] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | B01206 Archived ᱒᱐᱑᱙-᱐᱔-᱐᱗ at the Wayback Machine. | ||
| 3535004 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.070 | ||
| EC Number | 231-977-3 | ||
| 303 | |||
| KEGG | |||
| MeSH | Hydrogen+sulfide | ||
PubChem <abbr title="<nowiki>Compound ID</nowiki>">CID
|
|||
| RTECS number | MX1225000 | ||
| UNII |
| ||
| UN number | 1053 | ||
CompTox Dashboard (<abbr title="<nowiki>U.S. Environmental Protection Agency</nowiki>">EPA)
|
|||
| |||
| |||
| Properties | |||
| H2S | |||
| Molar mass | 34.08 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Pungent, like that of rotten eggs | ||
| Density | 1.363 g dm−3 | ||
| Melting point | −82 °C (−116 °F; 191 K) | ||
| Boiling point | −60 °C (−76 °F; 213 K) | ||
| 4 g dm−3 (at 20 °C) | |||
| Vapor pressure | 1740 kPa (at 21 °C) | ||
| Acidity (pKa) | 7.0[᱒][᱓] | ||
| Conjugate acid | Sulfonium | ||
| Conjugate base | Bisulfide | ||
| −25.5·10−6 cm3/mol | |||
Refractive index (nD)
|
1.000644 (0 °C)[᱔] | ||
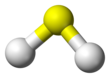
| Structure | |||
| C2v | |||
| Bent | |||
| 0.97 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
1.003 J K−1 g−1 | ||
Std molar
entropy (S |
206 J mol−1 K−1[᱕] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−21 kJ mol−1[᱕] | ||
| Hazards | |||
| Main hazards | Flammable and highly toxic | ||
EU classification (DSD) (outdated)
|
|||
| R-phrases (outdated) | R12, R26, R50 | ||
| S-phrases (outdated) | (S1/2), S9, S16, S36, S38, S45, S61 | ||
| NFPA 704 | 
<span style="color:black;" title="Flammability code 4: Will rapidly or completely vaporize at normal atmospheric pressure and temperature, or is readily dispersed in air and will burn readily. Flash point below 23 °C (73 °F). E.g., propane">4</span>
<span style="color:black;" title="Health code 4: Very short exposure could cause death or major residual injury. E.g., VX gas">4</span>
<span style="color:black;" title="Reactivity code 0: Normally stable, even under fire exposure conditions, and is not reactive with water. E.g., liquid nitrogen">0</span> | ||
| Flash point | −82.4 °C (−116.3 °F; 190.8 K) [᱖] | ||
Autoignition<br><br>temperature
|
232 °C (450 °F; 505 K) | ||
| Explosive limits | 4.3–46% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible)
|
C 20 ppm; 50 ppm [10-minute maximum peak][᱘] | ||
REL (Recommended)
|
C 10 ppm (15 mg/m3) [10-minute][᱘] | ||
IDLH (Immediate danger)
|
100 ppm[᱘] | ||
| Related compounds | |||
Related hydrogen chalcogenides
|
| ||
Related compounds
|
Phosphine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox<span typeof="mw:Entity"> </span>references | |||
ᱦᱟᱭᱰᱨᱚᱡᱟᱱ ᱥᱟᱞᱯᱷᱟᱭᱤᱰ ᱢᱤᱫ ᱨᱟᱥᱟᱭᱱᱤᱠ ᱭᱚᱣᱜᱤᱠ ᱥᱟᱶ ᱯᱷᱚᱨᱢᱩᱞᱟ H
2S ᱠᱟᱱᱟ᱾
H
2S ᱫᱚ ᱵᱷᱚᱞᱠᱟᱱᱤᱠ ᱜᱮᱥ, ᱱᱮᱪᱩᱨᱟᱞ ᱜᱮᱥ ᱟᱨ ᱫᱟᱜ ᱨᱮᱱᱟᱜ ᱯᱷᱮᱰᱟᱛ ᱠᱷᱚᱱ ᱧᱟᱢᱚᱜᱼᱟ ᱾ [᱙] ᱢᱟᱹᱱᱢᱤᱭᱟᱜ ᱦᱚᱲᱢᱚ ᱦᱚᱸ ᱱᱟᱥᱮ ᱩᱰᱤᱡ H
2S ᱮ ᱛᱮᱭᱟᱨᱮᱫᱼᱟ ᱟᱨ ᱥᱤᱜᱽᱱᱟᱞᱤᱝ ᱢᱚᱞᱤᱠᱩᱞ (signaling molecule) ᱞᱮᱠᱟᱛᱮ ᱵᱮᱵᱷᱟᱨᱮᱫᱼᱟ ᱾[᱑᱐]
ᱱᱚᱶᱟ ᱫᱚ ᱵᱮᱨᱚᱝ ᱪᱟᱞᱠᱚᱡᱮᱱ ᱦᱟᱭᱰᱨᱟᱭᱤᱰ (chalcogen hydride) ᱜᱮᱥ ᱥᱟᱶᱛᱮ ᱥᱮᱭᱟ ᱥᱤᱢᱵᱤᱞᱤ ᱞᱮᱠᱟ ᱥᱚ ᱣᱟᱱᱟ ᱾ ᱱᱚᱶᱟ ᱫᱚ ᱟᱹᱰᱤ ᱵᱤᱥ ᱜᱮᱭᱟ, ᱠᱨᱚᱡᱤᱵᱽ ᱟᱨ ᱞᱟᱜᱮ ᱟᱛᱟᱨᱚᱜᱼᱟ ᱾[᱑᱑]
ᱥᱩᱭᱰᱤᱥ ᱨᱤᱱᱤᱡ ᱥᱟᱬᱮᱥᱤᱭᱟᱹ ᱠᱟᱨᱞ ᱣᱤᱞᱦᱮᱞᱢ ᱥᱮᱞᱮ (Carl Wilhelm Scheele) ᱱᱚᱶᱟ ᱠᱮᱢᱤᱠᱟᱞ ᱠᱚᱢᱯᱚᱡᱤᱥᱚᱱ ᱫᱚᱭ ᱧᱟᱢ ᱚᱰᱚᱠ ᱞᱮᱫ ᱛᱟᱦᱮᱸᱫ ᱑᱗᱗᱗ ᱥᱟᱞᱮ ᱨᱮ ᱾
ᱜᱩᱱ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱥᱤᱨᱡᱟᱹᱣ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- FeS + 2 HCl → FeCl2 + H2S
- ᱱᱤᱴᱨᱮᱴ ᱢᱮᱥᱟ
- ᱠᱟᱞᱥᱤᱭᱚᱢ ᱱᱟᱭᱴᱨᱮ ᱫᱚ ᱞᱤᱸᱡᱤᱱ ᱫᱟᱜ ᱨᱮ ᱦᱟᱭᱰᱨᱚᱜᱮᱱ ᱥᱚᱞᱯᱷᱟᱭᱤᱰ ᱛᱮᱭᱟᱨᱚᱜ ᱠᱷᱚᱱ ᱵᱟᱧᱪᱟᱣ ᱞᱟᱹᱜᱤᱫ ᱵᱮᱵᱷᱟᱨ ᱜᱟᱱᱚᱜᱼᱟ ᱾
ᱯᱷᱩᱭᱤᱞ ᱜᱮᱥ ᱠᱷᱚᱱ ᱚᱪᱚᱜ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱱᱚᱸᱰᱮ ᱦᱚᱸ ᱧᱮᱞᱢᱮ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱥᱟᱹᱠᱷᱭᱟᱹᱛ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- ↑ "Hydrogen Sulfide - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ Perrin, D.D. (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution (2nd ed.). Oxford: Pergamon Press.
- ↑ Bruckenstein, S.; Kolthoff, I.M., in Kolthoff, I.M.; Elving, P.J. Treatise on Analytical Chemistry, Vol. 1, pt. 1; Wiley, NY, 1959, pp. 432–433.
- ↑ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 978-0-07-049439-8.
- ↑ ᱕.᱐ ᱕.᱑ Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ↑ "Hydrogen sulfide" Archived ᱒᱐᱑᱓-᱐᱕-᱓᱐ at the Wayback Machine.. npi.gov.au.
- ↑ ᱗.᱐ ᱗.᱑ "Hydrogen sulfide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ ᱘.᱐ ᱘.᱑ ᱘.᱒ NIOSH Pocket Guide to Chemical Hazards. "#0337". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Hydrogen Sulphide In Well Water". Retrieved 4 September 2018.
- ↑ Bos, E. M; Van Goor, H; Joles, J. A; Whiteman, M; Leuvenink, H. G (2015). "Hydrogen sulfide: Physiological properties and therapeutic potential in ischaemia". British Journal of Pharmacology. 172 (6): 1479–1493. doi:10.1111/bph.12869. PMC 4369258. PMID 25091411.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
{{cite book}}: Cite has empty unknown parameter:|name-list-format=(help); Invalid|ref=harv(help)