ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ
 | |||||||||||||||
| ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /mæɡˈniːziəm/ | ||||||||||||||
| Appearance | shiny grey solid | ||||||||||||||
| Standard atomic weight Ar, std(Mg) | [24.304, 24.307] conventional: 24.305 | ||||||||||||||
| ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ in the periodic table | |||||||||||||||
| |||||||||||||||
| Atomic number (Z) | 12 | ||||||||||||||
| Group | group n/a | ||||||||||||||
| Period | [[Period {{{period}}} element|period {{{period}}}]] | ||||||||||||||
| Block | [[{{{block}}}-block]] | ||||||||||||||
| Element category | Alkaline earth metal | ||||||||||||||
| Electron configuration | [Ne] 3s2 | ||||||||||||||
Electrons per shell | 2, 8, 2 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase at STP | solid | ||||||||||||||
| Melting point | 923 K (650 °C, 1202 °F) | ||||||||||||||
| Boiling point | 1363 K (1091 °C, 1994 °F) | ||||||||||||||
| Density when liquid (at m.p.) | 1.584 g/cm3 | ||||||||||||||
| Heat of fusion | 8.48 kJ/mol | ||||||||||||||
| Heat of | 128 kJ/mol | ||||||||||||||
| Molar heat capacity | 24.869[᱑] J/(mol·K) | ||||||||||||||
pressure
| |||||||||||||||
| Atomic properties | |||||||||||||||
| Oxidation states | +1,[᱒] +2 (a strongly basic oxide) | ||||||||||||||
| Electronegativity | Pauling scale: 1.31 | ||||||||||||||
| energies |
| ||||||||||||||
| Atomic radius | empirical: 160 pm | ||||||||||||||
| Covalent radius | 141±7 pm | ||||||||||||||
| Van der Waals radius | 173 pm | ||||||||||||||
| Other properties | |||||||||||||||
| Natural occurrence | primordial | ||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | ||||||||||||||
| Speed of sound thin rod | 4940 m/s (at r.t.) (annealed) | ||||||||||||||
| Thermal conductivity | 156[᱓] W/(m·K) | ||||||||||||||
| Electrical resistivity | 43.9[᱔] nΩ·m (at 20 °C) | ||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||
| Magnetic susceptibility | +13.1×10−6 cm3/mol (298 K)[᱕] | ||||||||||||||
| Young's modulus | 45 GPa | ||||||||||||||
| Shear modulus | 17 GPa | ||||||||||||||
| Bulk modulus | 35.4[᱖] GPa | ||||||||||||||
| Poisson ratio | 0.290 | ||||||||||||||
| Mohs hardness | 1–2.5 | ||||||||||||||
| Brinell hardness | 44–260 MPa | ||||||||||||||
| CAS Number | 7439-95-4 | ||||||||||||||
| History | |||||||||||||||
| Naming | after Magnesia, Greece[᱗] | ||||||||||||||
| Discovery | Joseph Black (1755[᱗]) | ||||||||||||||
| First isolation | Humphry Davy (1808[᱗]) | ||||||||||||||
| |||||||||||||||
ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱫᱚ ᱢᱤᱫ ᱠᱮᱢᱤᱠᱟᱞ ᱮᱞᱤᱢᱮᱱᱴ ᱠᱟᱱᱟ; ᱱᱚᱣᱟ ᱨᱮᱭᱟᱜ ᱪᱤᱱᱦᱟᱹ ᱫᱚ Mg ᱟᱨ ᱮᱴᱳᱢᱤᱠ ᱱᱚᱢᱵᱚᱨ ( atomic number) ᱑᱒ ᱠᱟᱱᱟ᱾ ᱱᱚᱣᱟ ᱮᱞᱤᱢᱮᱱᱴ ᱫᱚ ᱪᱟᱢᱠᱤᱞᱟ ᱠᱟᱵᱽᱨᱟ ᱧᱚᱜ ᱞᱮᱠᱟ ᱧᱮᱞᱚᱜᱼᱟ ᱟᱨ ᱱᱚᱣᱟ ᱨᱮᱫᱚ ᱞᱚᱣ (low) ᱰᱮᱱᱥᱤᱴᱤ ᱢᱮᱱᱟᱜᱼᱟ᱾ ᱞᱚᱣ ᱢᱮᱞᱴᱤᱝ ᱯᱚᱭᱤᱱᱴ ᱟᱨ ᱪᱮᱛᱟᱱ ᱠᱮᱢᱤᱠᱟᱞ ᱨᱤᱭᱮᱠᱴᱤᱵᱷᱤᱴᱤ ᱢᱮᱱᱟᱜᱼᱟ᱾
ᱠᱨᱮᱠᱴᱨᱤᱥᱴᱤᱠ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱯᱷᱤᱡᱤᱠᱟᱞ ᱯᱨᱚᱯᱟᱨᱴᱤᱡᱽ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- Mg (s′ + ᱒ H᱒O (g′ → Mg (OH′ ᱒ (aq′ + H᱒ (g′ + ᱑᱒᱐᱓.᱖ kJ/mol)
ᱠᱮᱢᱤᱠᱟᱞ ᱯᱨᱚᱯᱟᱨᱴᱤᱡᱽ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱳᱠᱥᱤᱰᱮᱥᱟᱱ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱳᱨᱜᱮᱱᱤᱠ ᱠᱮᱢᱮᱥᱴᱨᱤ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱥᱚᱞᱩᱭᱥᱚᱱ ᱨᱮ ᱪᱤᱱᱦᱟᱹ ᱾
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱯᱷᱚᱨᱚᱢ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱟᱞᱳᱭᱠᱚ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱜᱷᱟᱹᱞ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱮᱞᱳᱭᱠᱚ ᱨᱮ, ᱟᱭᱨᱟᱱ, ᱱᱤᱠᱮᱞ, ᱠᱳᱯᱟᱨ ᱥᱮ ᱠᱳᱵᱟᱞᱴ ᱠᱮᱴᱮᱡ ᱮᱠᱴᱤᱵᱷᱮᱴ ᱠᱳᱨᱳᱥᱟᱱ ᱛᱟᱦᱮᱱ ᱠᱟᱱᱟ᱾[᱙]
ᱦᱟᱭ-ᱴᱮᱢᱯᱨᱮᱪᱟᱨ ᱠᱨᱤᱯ ᱟᱨ ᱯᱷᱞᱟᱢᱮᱵᱤᱞᱤᱴᱤ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱠᱚᱢᱯᱟᱣᱩᱱᱰ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱟᱭᱥᱳᱴᱳᱯ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱮᱞᱩᱢᱤᱱᱤᱭᱟᱢ ᱞᱮᱠᱟ, ᱱᱩᱠᱞᱟᱭᱰ 26Mg ᱫᱚ ᱟᱭᱥᱳᱴᱳᱯᱤᱠ ᱜᱤᱭᱳᱞᱡᱤ ᱨᱮ ᱵᱮᱣᱦᱟᱨ ᱧᱟᱢ ᱟᱠᱟᱫᱟ ᱾ 26Mg ᱫᱚ 26Al ᱨᱮᱭᱟᱜ ᱢᱤᱫᱴᱟᱝ ᱨᱮᱰᱤᱣᱳᱡᱮᱱᱤᱠ ᱰᱚᱛᱚᱨ ᱯᱮᱠᱴᱚᱠ, ᱡᱟᱦᱟ ᱗᱑᱗,᱐᱐᱐ ᱥᱮᱨᱢᱟ ᱨᱮᱭᱟᱜ ᱢᱤᱫ ᱟᱫᱷᱟ ᱡᱤᱭᱚᱱ ᱾ ᱠᱟᱹᱪ ᱠᱟᱨᱵᱟᱱᱮᱥᱤᱥ ᱪᱚᱱᱰᱨᱟᱭᱤᱴᱢᱮᱴᱳᱨᱟᱭᱤᱴ ᱾ ᱨᱮ ᱥᱤ ᱮᱼᱮᱞᱼᱨᱮᱪ ᱥᱮᱞᱮᱫ ᱨᱮ ᱟᱹᱰᱤ ᱡᱟᱹᱥᱛᱤ ᱯᱚᱨᱤᱢᱟᱱ ᱨᱮ ᱒᱖ ᱮᱢᱡᱤ ᱨᱮᱭᱟᱜ ᱥᱟᱹᱵᱩᱫ ᱧᱮᱞ ᱧᱟᱢ ᱟᱠᱟᱱᱟ ᱾ ᱱᱚᱶᱟ ᱵᱮᱼᱵᱮᱲᱟᱱ ᱯᱚᱨᱤᱢᱟᱱ ᱨᱮᱭᱟᱜ ᱚᱡᱮ ᱫᱚ ᱦᱩᱭᱩᱜ ᱠᱟᱱᱟ ᱥᱮᱞᱮᱫ ᱨᱮ ᱱᱚᱶᱟ ᱨᱮᱭᱟᱜ ᱢᱩᱲᱩᱫ 26Al26Al ᱜᱟᱹᱛᱤᱞ ᱟᱨ ᱵᱤᱰᱟᱹᱣᱤᱭᱟᱹ ᱠᱚ ᱢᱩᱪᱟᱹᱫ ᱨᱮ ᱞᱟᱹᱭ ᱟᱠᱟᱫᱟ ᱡᱮ ᱒᱖Al ᱜᱚᱫᱚᱜ ᱞᱟᱦᱟ ᱱᱚᱶᱟ ᱞᱮᱠᱟᱱ ᱢᱮᱴᱳᱨᱟᱭᱤᱴ ᱫᱚ ᱥᱩᱨᱟᱜ ᱱᱮᱵᱩᱞᱟ ᱨᱮ ᱵᱮᱱᱟᱣ ᱞᱮᱱᱟ ᱾ ᱱᱚᱶᱟᱠᱚ ᱫᱚ ᱫᱚ ᱥᱩᱵᱚᱨ ᱜᱚᱫᱚᱢ ᱨᱮᱭᱟᱜ ᱢᱟᱨᱮᱱ ᱡᱤᱱᱤᱥ ᱠᱚ ᱢᱩᱫᱽᱨᱮ ᱟᱨ ᱱᱚᱶᱟ ᱨᱮᱭᱟᱜ ᱮᱛᱚᱦᱚᱵ ᱱᱟᱜᱟᱢ ᱵᱟᱨᱮᱛᱮ ᱥᱟᱧᱪᱟᱣ ᱛᱮ ᱢᱮᱱᱟᱜ ᱛᱚᱛᱛᱟ ᱾
ᱵᱮᱱᱟᱣ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱳᱠᱭᱩᱨᱮᱱᱥ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- MgCl2 + Ca (OH᱒ → Mg (OH ᱒ + CaCl2)
ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱦᱟᱭᱰᱨᱚᱠᱥᱟᱭᱰ (ᱵᱽᱨᱩᱥᱟᱭᱤᱴ) ᱫᱟᱜ ᱨᱮ ᱵᱟᱹᱲᱤᱡ ᱥᱳᱞᱭᱩᱵᱟᱞ ᱢᱮᱱᱟᱜᱼᱟ ᱟᱨ ᱱᱚᱣᱟ ᱫᱚ ᱯᱷᱤᱞᱴᱟᱨᱮᱥᱟᱱ ᱦᱚᱛᱮᱛᱮ ᱡᱟᱢᱟ ᱦᱩᱭ ᱫᱟᱲᱮᱭᱟᱜᱼᱟ᱾ ᱱᱚᱣᱟ ᱫᱚ ᱦᱟᱭᱰᱨᱠᱞᱳᱨᱤᱠ ᱮᱥᱤᱰ ᱥᱟᱶᱛᱮ ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱠᱞᱳᱨᱟᱭᱤᱰ ᱨᱮᱭ ᱨᱤᱭᱮᱠᱥᱟᱱ ᱮᱫᱟ᱾[᱑᱐]
- Mg (OH᱒ + ᱒ HCl → MgCl2 + ᱒ H᱒O) H2O
ᱢᱮᱜᱽᱤᱥᱤᱭᱟᱢ ᱠᱞᱳᱨᱟᱭᱤᱰ ᱠᱷᱚᱱ, ᱤᱞᱮᱠᱴᱨᱚᱞᱟᱭᱥᱤᱥ ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱵᱮᱱᱟᱜ ᱠᱟᱱᱟ᱾[᱑᱑]
ᱟᱨᱡᱟᱣ ᱨᱮᱭᱟᱜ ᱯᱟᱨᱤᱢᱟᱱ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱦᱩᱭᱯᱤᱫᱡᱚᱱ ᱟᱨ ᱵᱳᱞᱡᱟᱱᱳ ᱦᱚᱨᱟ ᱾
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱰᱟᱣᱩ ᱦᱚᱨᱟ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- Mg᱒ + (aq) + CaO (s) + H᱒O (l) → Ca᱒ + (ac) + Mg (OH) ᱒ (s)
ᱠᱟᱨᱵᱳᱛᱷᱟᱨᱢᱤᱠ ᱦᱚᱨᱟ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]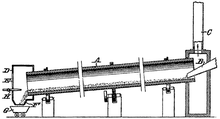
ᱳᱭᱮᱹᱥᱮᱹᱰ ᱦᱚᱨᱟ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱨᱤᱮᱠ ᱦᱚᱨᱟ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱱᱟᱜᱟᱢ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱵᱮᱣᱦᱟᱨ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱢᱮᱜᱽᱱᱤᱥᱤᱭᱟᱢ ᱢᱮᱛᱟᱞ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱩᱰᱟᱹᱱ ᱜᱟᱹᱰᱤ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱳᱴᱳᱢᱳᱴᱤᱵᱷ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱡᱤᱭᱚᱱᱟᱱ ᱮᱱᱮᱢ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱱᱭᱩᱴᱨᱤᱥᱟᱱ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱡᱚᱢᱟᱜ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱡᱚᱢᱟᱜ ᱨᱮᱭᱟᱜ ᱯᱚᱨᱟᱢᱚᱥ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱥᱟᱯᱞᱤᱢᱮᱱᱴᱮᱥᱟᱱ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]ᱢᱮᱴᱟᱵᱳᱞᱤᱡᱽᱢ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]
ᱛᱟᱹᱞᱤᱠᱟ ᱨᱮ ᱞᱟᱹᱭ ᱟᱠᱟᱱᱟ
[ᱥᱟᱯᱲᱟᱣ | ᱯᱷᱮᱰᱟᱛ ᱥᱟᱯᱲᱟᱣ]- ↑ Rumble, p. 4.61
- ↑ Bernath, P. F.; Black, J. H.; Brault, J. W. (1985). "The spectrum of magnesium hydride" (PDF). Astrophysical Journal. 298: 375. Bibcode:1985ApJ...298..375B. doi:10.1086/163620.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (help) - ↑ Rumble, p. 12.137
- ↑ Rumble, p. 12.28
- ↑ Rumble, p. 4.70
- ↑ Gschneider, K. A. (1964). Physical Properties and Interrelationships of Metallic and Semimetallic Elements. Solid State Physics. Vol. 16. p. 308. doi:10.1016/S0081-1947(08)60518-4. ISBN 9780126077162.
- ↑ ᱗.᱐ ᱗.᱑ ᱗.᱒ Rumble, p. 4.19
- ↑ ᱘.᱐ ᱘.᱑ ᱘.᱒ ᱘.᱓ Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ↑ Birbilis, N.; Williams, G.; Gusieva, K.; Samaniego, A.; Gibson, M. A.; McMurray, H. N. (2013). "Poisoning the corrosion of magnesium". Electrochemistry Communications. 34: 295–298. doi:10.1016/j.elecom.2013.07.021.
- ↑ "Magnesium processing | Techniques & Methods | Britannica". www.britannica.com (in ᱟᱝᱜᱽᱨᱮᱡᱤ). Retrieved 2024-05-04.
- ↑ "Magnesium metal is produced by the electrolysis of molten magnesi... | Channels for Pearson+". www.pearson.com (in ᱟᱝᱜᱽᱨᱮᱡᱤ). Retrieved 2024-05-04.
ᱛᱩᱢᱟᱹᱞ ᱦᱩᱲᱟᱹᱜ:<ref> tags exist for a group named "lower-alpha", but no corresponding <references group="lower-alpha"/> tag was found
- CS1 errors: unsupported parameter
- CS1 ᱟᱝᱜᱽᱨᱮᱡᱤ-language sources (en)
- Articles with short description
- Short description is different from Wikidata
- Pages using infobox element with unknown parameters
- ᱢᱮᱴᱟᱞ
- ᱮᱴᱳᱢᱤᱠ ᱱᱚᱢᱵᱚᱨ
- ᱮᱴᱚᱢᱤᱠ ᱮᱞᱮᱞ
- ᱯᱟᱨᱢᱟᱱᱩ ᱥᱟᱝᱠᱷᱭᱟ
- ᱥᱟᱦᱴᱟ ᱨᱮ ᱥᱟᱹᱠᱷᱭᱟᱹᱛ ᱠᱚ ᱵᱷᱩᱞ ᱜᱮ ᱢᱮᱱᱟᱜᱼᱟ
- Pages with reference errors that trigger visual diffs

